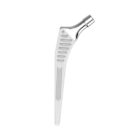
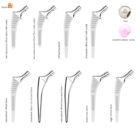
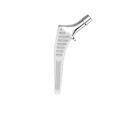
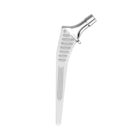
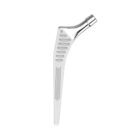
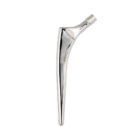
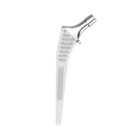
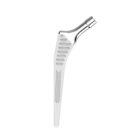
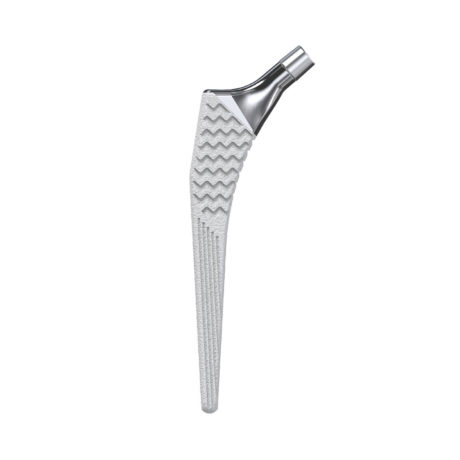
HYPE® Stems
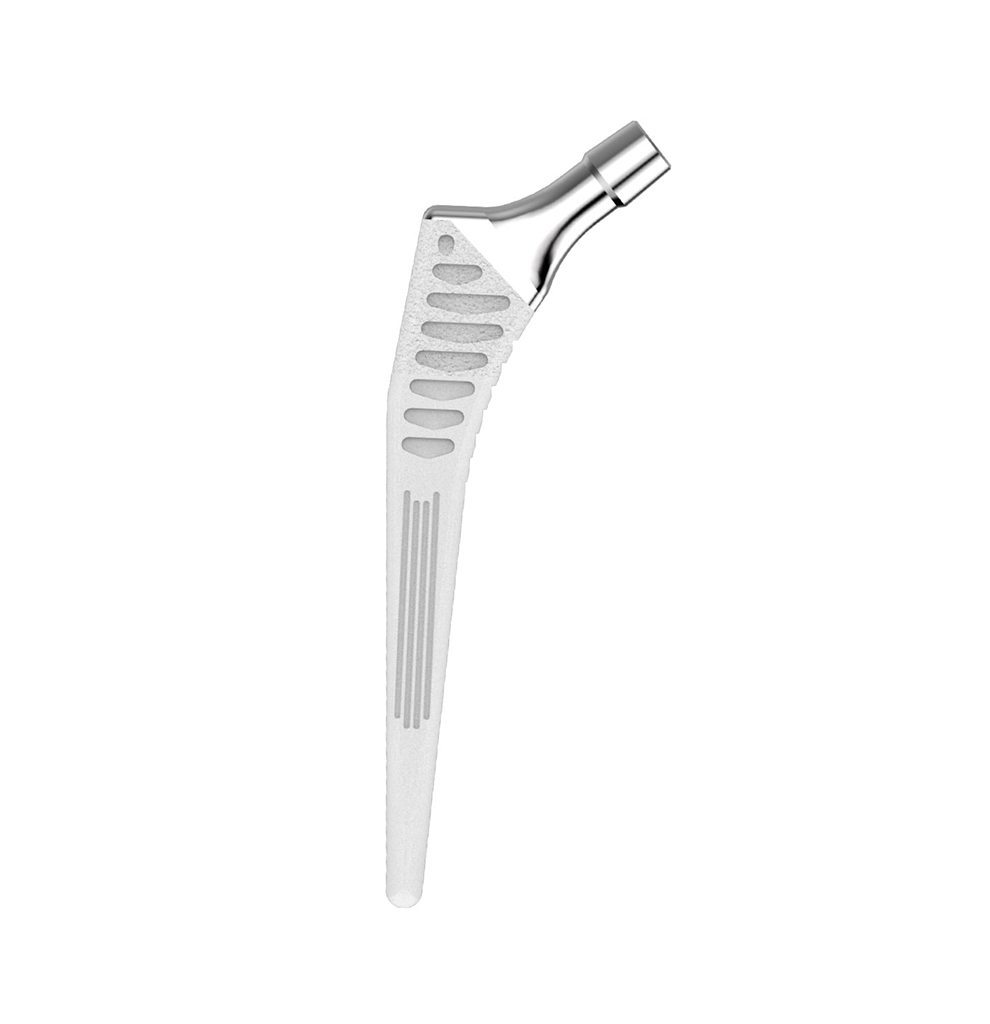
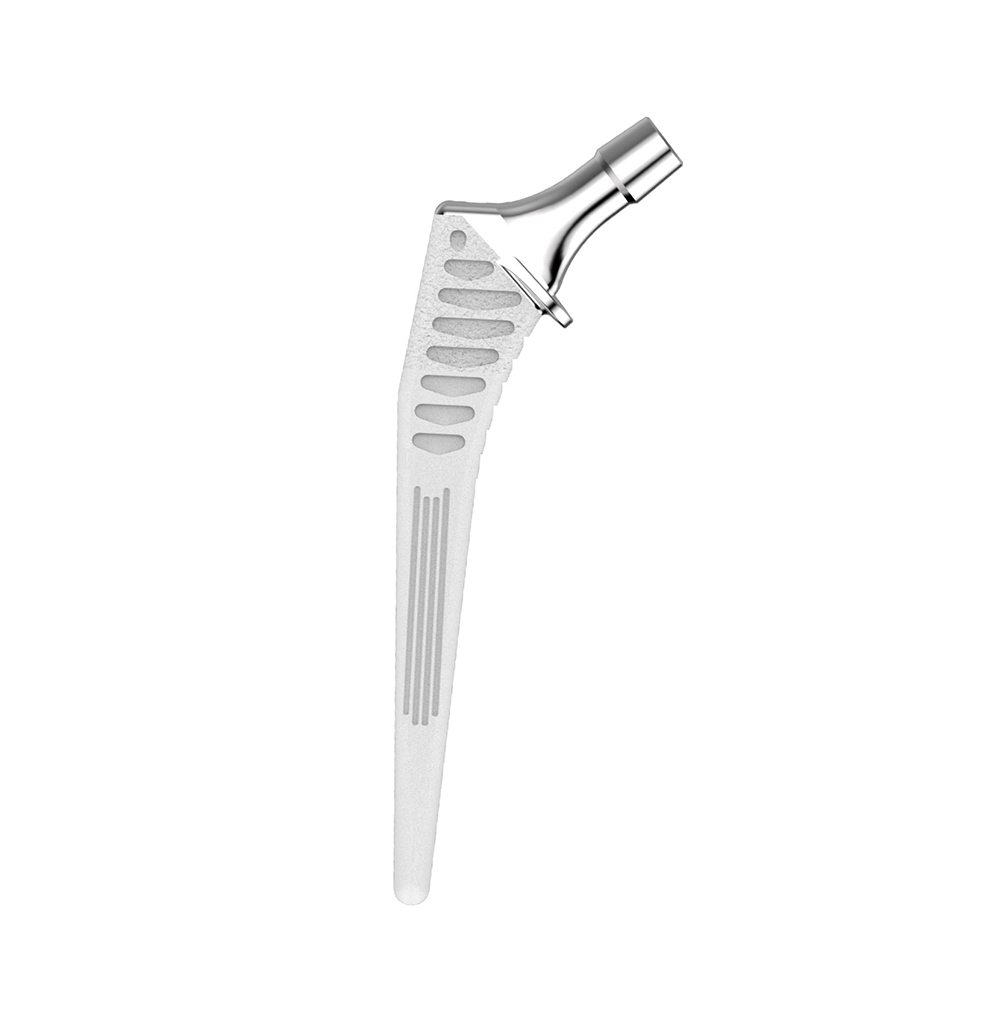
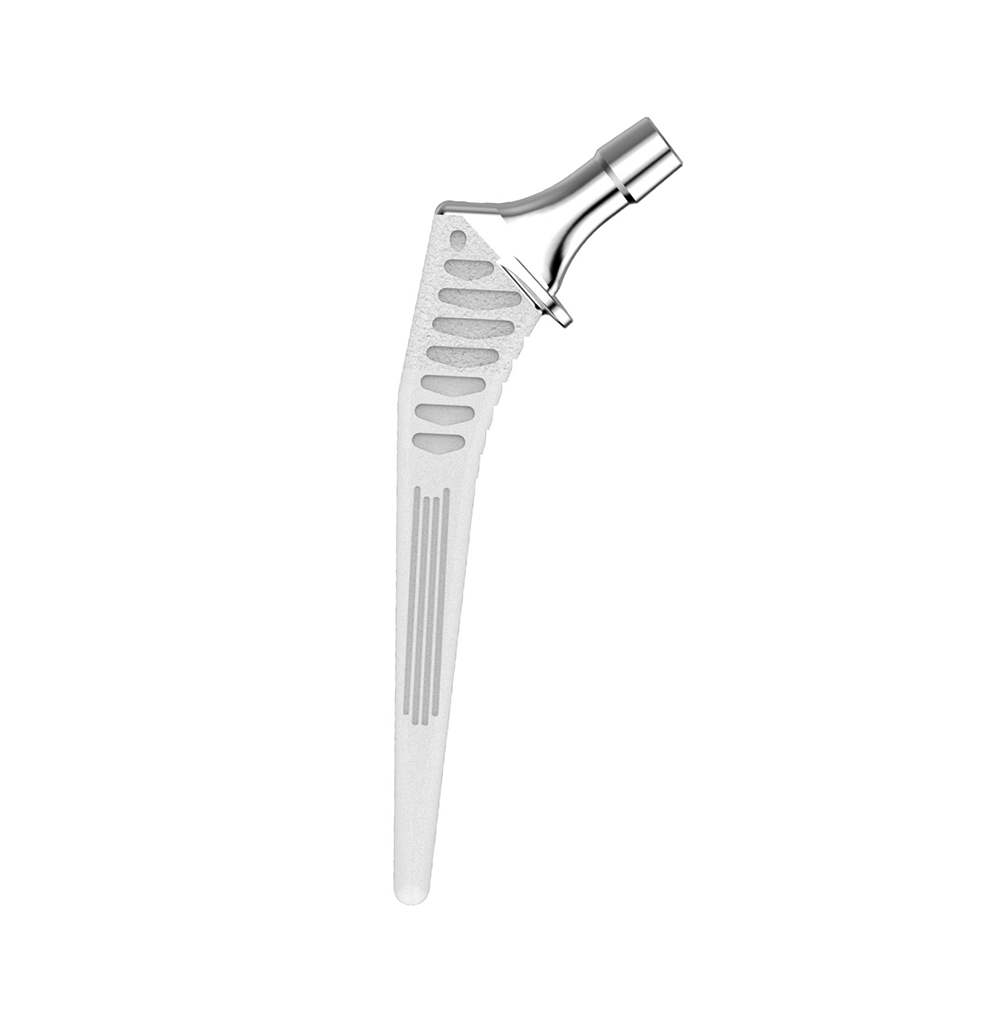
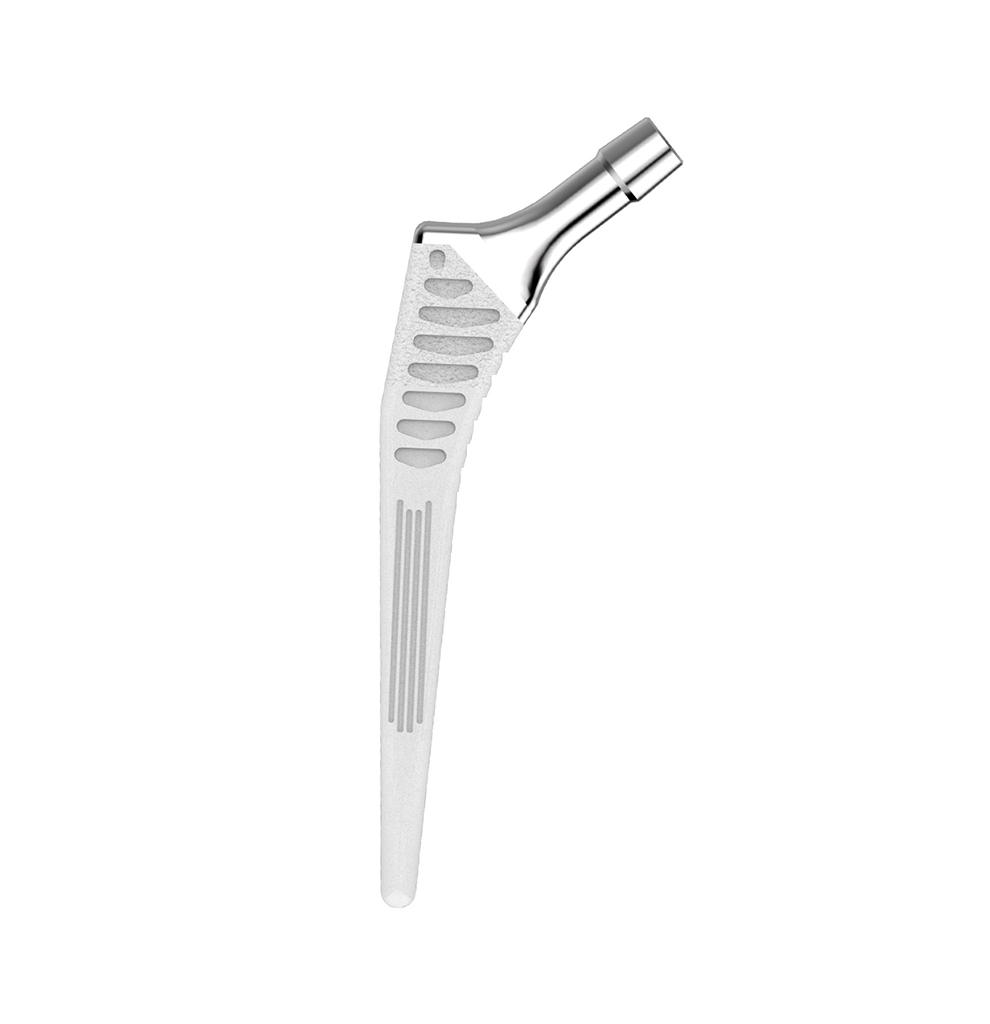
The Hype® range of femoral stem is a complete range of self locking stems dedicated to primary total hip arthroplasty. These stems have a 12/14 (5°43’) taper.
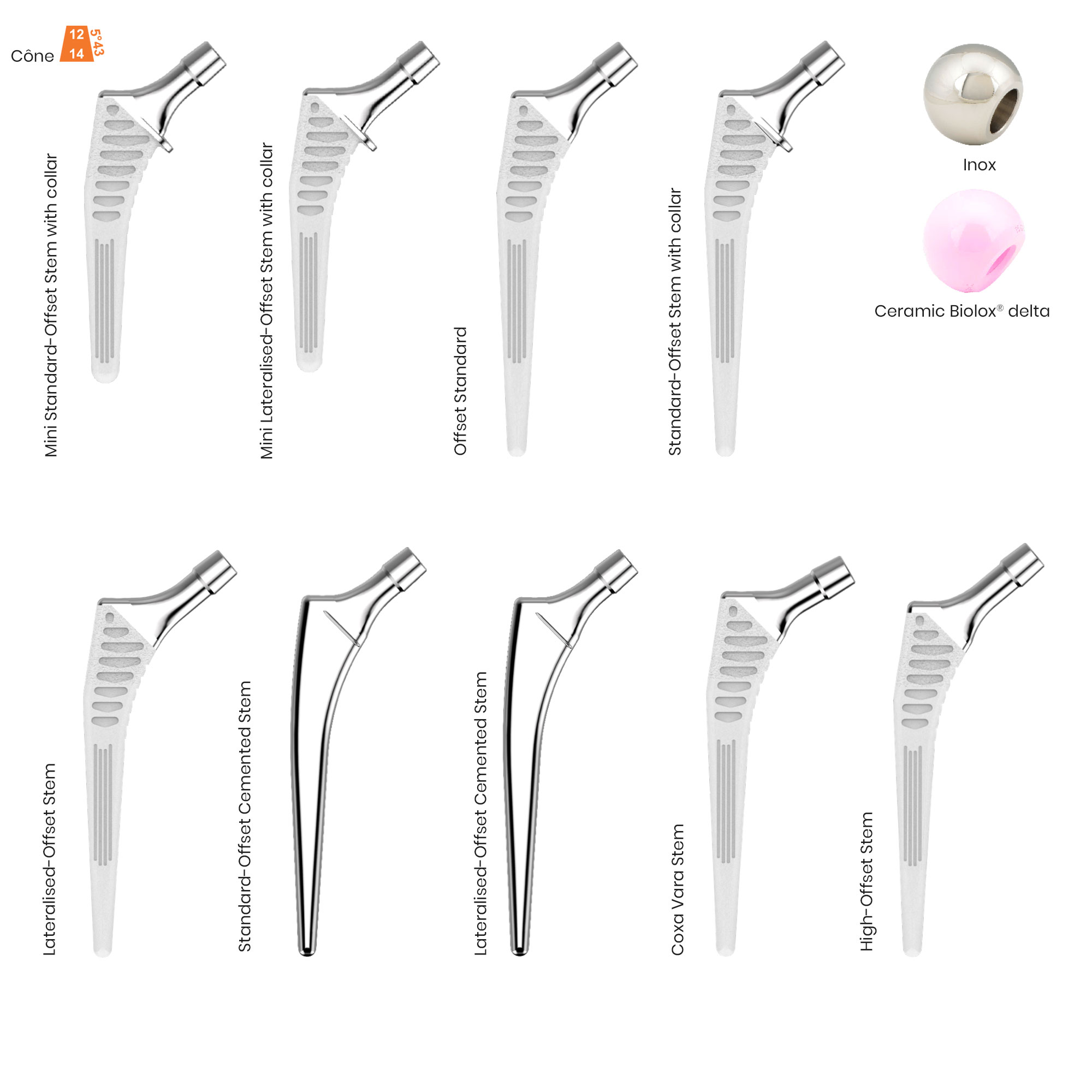
A total of 9 stems are available, including two short stems, in standard and lateralised offsets (6 mm lateralisation compared with standard stems), and also two cemented stems.
Characteristics and materials
Characteristics :
- Stems are available in short and standard, cementless and cemented, collared and collarless versions.
- Homothetic size progression of stem collars (except for the coxa vara stem).
- CCD angle : 120° for the coxa vara stem and 130° for other stems in the range.
- 12/14 (5°43’’) taper which needs to be used with SERF “I”, “D”, or “C” heads with the same 12/14 taper.
Materials :
- Cemented stems : stainless steel.
- Cementless stems : titanium alloy with titanium spray + dual coating made of titanium spray and HA.
- Femoral heads: “I” made from stainless steel and “D” made from Biolox® delta ceramic. Beside and only for US market femoral heads “C” in cobalt-chromium is also available.
Instruments
- The same instrumentation set for all standard-length stems.
- Additional set for Hype® Mini stems.
- A wide choice of broach handles for the different approaches : posterior, posterolateral, anterior and anterolateral.
Indications outside us
Hype® stems are indicated in the following cases :
- First-stage surgery;
- Advanced joint destruction resulting from rheumatoid or post-traumatic arthritis;
- Primary or secondary osteoarthritis, displaced subcapital and transcervical fracture as well as for Paprosky type I to IIB bone defects.
Hype® mini implants are more specifically indicated for anterior approaches or when the use of a shorter stem is more appropriate.
Contraindications of Hype® stems are as follows :
- Acute or chronic local or systemic infection (cardiopathy, decompensated diabetes, continuous haemodialysis, reduced immune response, etc.);
- severe muscular, neurological or vascular deficiencies affecting the extremity concerned;
- Bbone destruction or loss, or poor bone quality likely to affect implant stability, severe osteoporosis, major deformity of the joint to be replaced, local bone tumours;
- all related conditions which could compromise the function or implanting of the prosthesis;
- systemic or metabolic disorders;
- mental incapacity of patients to understand the surgeon’s instructions;
- drug addiction, alcohol, tobacco or medicine abuse;
- local bone tumours;
- obesity, excess weight, high level of physical activity, intense exercise, after a fall;
- size-1 Hype® SCC and Hype® SCS stems must only be used in patients weighing less than 55 kg;
- size-2 Hype® SCC, Hype® SCS and Hype® SCL stems must only be used in patients weighing less than 70 kg;
- hype® Mini stems are not recommended for use in obese or overweight patients;
- the size-2 mini Hype® stem is not recommended for use in patients weighing 70 kg or over;
- the size-2 Hype® SCV stem must only be used in patients weighing less than 70 kg.
Indications for us market
Hype® stems are indicated in the following cases :
-
-
- osteoarthritis;
- femoral neck fracture;
- osteonecrosis of the femoral head.
-
Hype® SCS, SCC, SCL, SCV, SCC Mini, and SCLA Mini hip stems are intended for press-fit use.
Hype® mini implants are more specifically indicated for anterior approaches or when the use of a shorter stem is more appropriate.
Contraindications of Hype® stems are as follows :
-
-
- Infection of the concerned limb;
- any contraindication to surgery or anesthesia;
- severe muscular, neurological or vascular deficiencies affecting the limb concerned;
- destruction, loss or poor bone quality that could affect the stability of the implant, serious deformation of the joint that needs replacing;
- local bone tumours;
- any associated disorder that could compromise the function or the implementation of the prosthesis;
- obesity;
- intense activity or intensive;
- sports training.
-