NOVAE® Acetabular Cups
The Novae® range is based on the Dual Mobility concept invented in 1975 by Professor Gilles BOUSQUET (Saint-Etienne University Hospital) and Mr André RAMBERT, founder of SERF.
Dual Mobility is the combination of two principles in orthopaedics:
- Sir Charnley’s low friction concept: use of thick polyethylene with a small 22.2 mm head to reduce wear.
- The McKee-Farrar concept: use of a large head to reduce the risk of prosthesis dislocation and instability.
The Dual Mobility design is made of two bearing surfaces :
- Small articulation (or first mobility): articulation of the prosthesis head in the insert.
- Large articulation (or second mobility): articulation of the insert in the cup.
Characteristics and materials
The Novae® cup range includes 5 implants :
- Novae® SunFit TH is intended for cementless fixation due to its equatorial press-fit and dual coating.
- Novae® E TH has two pegs secured with fixing screws (one for the Novae® E TH) to complete bone anchoring.
- Novae® Coptos TH has two pegs secured with fixing screws (4 screws for the Coptos TH) to complete bone anchoring.
- Novae® Stick must be cemented to the bone and may be combined with the Novae® K E cross plate.
- Novae® K E cross plate.
Novae® implants are made from the materials listed below :
- Cementless cup: Stainless steel + dual coating made of titanium spray and HA.
- Cemented cup and fixing screw: Stainless steel.
- Pins: Alumina-coated stainless steel
- Novae® K E cross plate: Stainless steel and PMMA
- CI E liner: UHMWPE
- XPEO-E : Highly Crosslinked UHMWPE stabilized with an antioxidant (Vitamin E) – Approved for US market
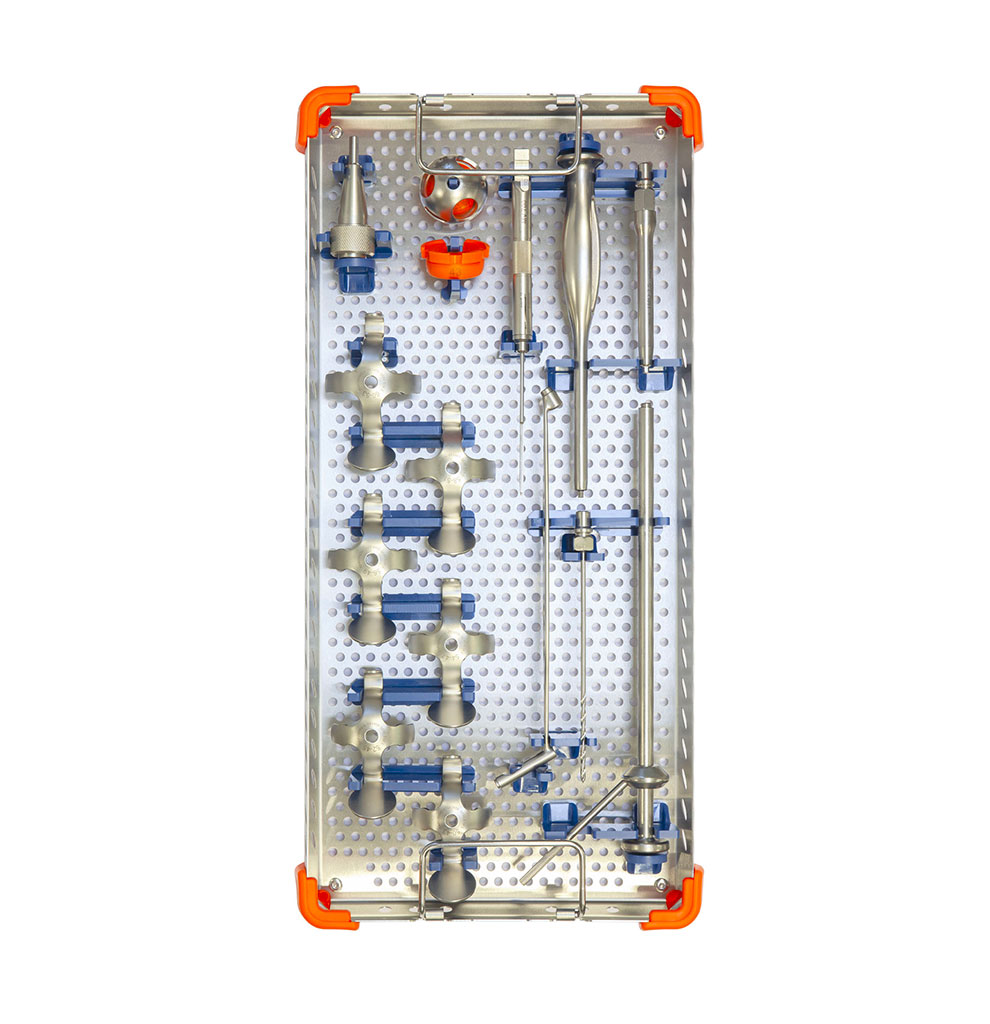
Instruments
- One instrumentation set common for all Novae® acetabular cups.
- Additional set for pegs and screw preparation for Novae® E TH and Coptos TH.
- Specific instrumentation for the Novae® K E cross plate.
Indications
Novae® cups are particularly indicated for patients with a high risk of dislocation and for patients over 65 years.
Indications of the Novae® cup range differ according to the implant :
Novae® Sunfit TH :
Acetabular cups combining a “Sunfit TH” and a “CI E” are intended for the treatment of the following conditions :
- Osteoarthritis of the hip, fractures of the femoral neck, osteonecrosis,
- Surgical revision (chronic dislocation, loosening, etc.) if the bone reconstruction allows, Paprosky type I, IIA and IIC bone defects.
Novae® E TH :
Acetabular cups combining a “Novae® E TH” and a “CI E” are intended for the treatment of the following conditions :
- Osteoarthritis of the hip, osteonecrosis,
- Surgical revision (including recurrent dislocation and loosening) if the bone reconstruction allows, Paprosky type I, IIA and IIC bone defects.
Coptos TH :
Acetabular cups combining a “Coptos TH” and a “CI E” are intended for the treatment of the following conditions :
- Surgical revision (including recurrent dislocation and loosening) if the bone reconstruction allows, with Paprosky type IIA, IIB and IIC.
Novae® Stick :
Acetabular cups combining a “Novae® Stick” cup and a CI E Liner are intended for treatment of the following conditions :
- Osteoarthritis of the hip, fractures of the femoral neck, periacetabular metastatic disease, patients with a risk of instability (neurological, tumour, revision, dearthrodesis, cognitive issues, etc.) that could compromise the primary stability.
- Particularly recommended in cases of osteoporotic bone
- Osteonecrosis
- Dysplasia
- Can be used for rehabilitation after severe Paprosky type IIIA and IIIB acetabular destruction, by cementing the cup in a fixed acetabular cage.
- Can be used alone (without a fixed acetabular cage), when other acetabular cups are not appropriate.
- Surgical revision (including chronic dislocation, loosening) if the bone reconstruction allows, Paprosky type IIIA and IIIB bone defects.
Novae® K E :
- Severe bone loss
- Revision arthroplasty
- Acetabular reconstruction
- Type 3A and 3B (Paprosky) bone defects
Contraindications of the Novae® cup range are as follows :
Contraindications relating to use of Novae® dual mobility cups and liners are local or systemic acute or chronic conditions (heart disease, uncompensated diabetes, regular haemodialysis, impairment of the immune system, etc.).
Contraindications of the Novae® KE range are as follows :
- Local or systemic acute or chronic infections (heart disease, uncompensated diabetes, regular hemodialysis, impairment of the immune system, etc.)
- Severe muscular, neurological or vascular deficiencies affecting the extremity concerned
- Destruction, loss or poor bone quality that could affect the stability of the implant, severe osteoporosis, serious deformation of the joint that needs replacing, local bone tumours
- Any associated disorder that could compromise the function or the implantation of the prosthesis
- Addiction to drugs, alcohol, smoking or medication
- A patient’s intellectual incapacity to understand the surgeon’s instructions
- Systemic or metabolic issues
- Local bone tumours,
- Obesity, excess weight, intense activity, intensive sports training, falls.